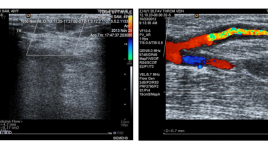
Nghiên cứu đặc điểm siêu âm lỗ dò động mạch quay - tĩnh mạch đầu có giảm lưu lượng ở bệnh nhân lọc máu chu kỳ
23/05/2020 11:02:13 | 0 binh luận
SUMMARY Objectives : Assessing the characteristics of radio - cephalic fistulas with insufficient flow volume in dialysis patients by Dupplex sonography. Subjects and methods : A cross - sectional study from 20 dialysis patients who have radio - cephalic fistulas with insufficient flow volume. Results: In 20 patients (13 males and 7 females), average age (43.7 ±1.18 years of age). Diameter of juxta-anastomotic orifice is 3.1± 0.9 mm in males and 2.7 ± 1.2 mm in females. Diameter of cephalic vein is 3.6 ± 0.9 mm in males and 2.8 ± 1.2 mm in females. Some hemodynamic indexs of fistular: PSV (89.55 ±101.61cm/s), ESV (51.80 ± 45.50 cm/s), RI 0.76 ± 0.23) and flow volume (130.95 ± 110.04 ml/minute). Some causes of insufficient flow volume: thrombosis 9/20 (45%), accessory veins (3/20 (15%), intimal hyperplasia 2/20 (10%), juxta-anastomotic stenosis due to thrombosis 1/20 (5%) and no cause found 5/20 (25%). Conclusions : Dupplex sonography can assess the flow volume of fistula and causes of insufficient flow volume. It is a useful tool for clinicians in following up the AV fistulas.

Nghiên cứu giá trị của sinh thiết dưới hướng dẫn siêu âm qua đường trực tràng trong chẩn đoán ung thư tuyến tiền liệt
31/03/2020 21:29:51 | 0 binh luận
Researching the value of biopsy under guideline of transrectal ultrasound in the diagnosis of Prostate cancer SUMMARY: Purpose: To describe the image of prostate cancer in transrectal ultrasound and to define the value of biopsy under guideline of transrectal ultrasound Materials and method: Prospective study on 190 patients with prostate biopsy in Viet Duc hospital from Sep/ 2013 to Aug/ 2014, of those 68 cases are cancer. Describe the transrectal ultrasound image and study the sensitivity, the specificity, the accuracy of biopsy under guideline of transrectal ultrasound. Results : Prostate cancer on the transrectal ultrasound has the image of rough margin 73.5%; unclear between internal and external zone 54.4%; irregular hyper angiogenesis 61.8%; disappear the image of fat around prostate 52.9%; node with hypo-echo 100%, hyper angiogenesis 89.2%, rough margin 56.1% and in the external 67.6%. The 10 speciment prostate biopsy by transrectal ultrasound have the sensitivity 94.7%; the specificity 100.0%; the accuracy 98.0% and low complication with the rate of un-complication of 64.7%. Conclusion: The prostate biopsy under guideline of transcretal ultrasound have high value in early diagnosis the prostate cancer, safely and less complication. Keywords: prostate biopsy, transcretal ultrasound.
Nghiên cứu ứng dụng kĩ thuật sinh thiết lõi qua thành ngực dưới hướng dẫn của siêu âm
02/04/2020 22:10:08 | 0 binh luận
Ultrasound-guided transthoracic core biopsy SUMMARY: Background: On the global scale, primary lung cancer is a major contributor to both cancer incidence and mortality. Definite diagnosis can be made from cytology or biopsy . A variety of techniques are available and helpful for the sampling include bronchoscopy, fine needle aspiration or image-guided biopsy, in which ultrasound-guided core biopsy of lung solid masses has been widely used as it is available, convenient and cost-effective. Material and method: A cross-sectional study was done on a sample of 77 individuals who had juxtapleural lung solid masses detected by Ultrasound or CT scan at Hue central hospital, from January-2010 to August-2011. All of these have undergone ultrasound-guided core biopsy. Objectives were to evaluate the efficacy, advantages and disadvantages of the technique. Results: The successful rate of single sampling was 89.6%. Five patients had twice sampling which raised the successful rate up to 94.8%, from which 89.6% were indicated malignant and the rest 5.2% were benign lesions. Advantages were multiple sampling (>2), supine and inclination position, skin surface-lesion distance less than 3 cm, pleura-lesion interface over 2 cm, lesion’s depth over 2 cm. Disadvantages include multiple sampling, lesion’s depth over 3 cm, FEV1/FVC < 0.7, needle size (<17G), prolonged procedure (>25 minutes). Rate of complication was 14.6%, in which pneumothorax was the most likely (4.9%), but no pleural drainage needed. Conclusion: ultrasound-guided core biopsy appeared safe, effective, non radiation exposure, cost-saving, simple and applicable in clinical setting. Key words: ultrasound-guided biopsy, lung solid mass.
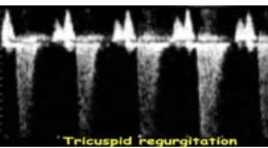
Gía trị chẩn đoán lệch bội nhiễm sắc thể trên thai kỳ nguy cơ cao của nhưng dấu hiệu siêu âm mới ở ba tháng đầu
02/04/2020 21:09:04 | 0 binh luận
Chromosomal defects’ evaluation with new ultrasound markers in first trimester having high genetic risk pregnancies SUMMARY: Objective: To determine sensitivity (Se), specificity (Sp), false positive rate (FPR), positive predictive value (PPV) of new ultrasound markers on high genetic risk pregnancies between weeks 11 and 14. Before CVS procedure, the new ultrasound markers nasal bone(NB), DV and TR were assessed. We determined diagnostic evaluation of new ultrasound markers based on ultrasound findings and genetic results from CVS – gold standard. Results: NB marker: Se: 60%, Sp: 97.1%, FPR: 2.9% for T21. Se: 60%, Sp: 94.5%, FPR: 5.5% for T18. Se: 50%, Sp: 93.6%, FPR: 6,4% for T13. Se: 100%, Sp: 92.9%, FPR::7,1% forTurner syndrome. DV marker: Se: 70%, Sp: 96.2,1%, FPR: 3.8% for T21. Sens: 60%, Spec: 92.7%, FPR: 7.3% for T18. Se: 50%, Sp: 91.8%, FPR: 8.2% forT13. Se: 100%, Sp: 91.1%, FPR: 8.9% forTurner syndrome. TR marker: Sens: 60%, Sp 96.1%, FPR: 3.9% for T21. Se: 40%, Sp: 92.7%, FPR: 73%, for T18. Se: 50%, Sp: 92.7%, FPR: 7.3% for T13. Se: 100%, Sp: 92%, FPR: 8.0% for Turner syndrome. Combine 3 new markers for detecting chromosome defects: Increase Se, Sp and decrease FPR.Se: 60%, Sp: 97,1%, FPR: 2,9%. Reducing 73% placental biopsies… Conclusion: New ultrasound markers in the first trimester improved diagnostic evaluation of chromosome defects and avoiding unnecessary placental biopsy. Key words: Chorionic villus sampling, nasal bone, ductus venosus, tricuspid flow.
Bước đầu nghiên cứu siêu âm đàn hồi mô tuyến giáp ở người bình thường bằng phương pháp tạo hình và đo vận tốc sóng biến dạng qua kĩ thuật ARFI
02/04/2020 20:59:31 | 0 binh luận
Preliminary studies on ultrasonic elastic tissue in the normal thyroid with the method of measuring shape and value velocity of shear wave by using a acoustic radiation force impulse image technology (ARFI) SUMMARY: Objectives: Measure the reference value of average velocity of shear wave in healthy healthy thyroid tissue by using acoustic radiation force impulse imaging. Methods: Evaluate 49 healthy volunteers with normal thyroid: 27 women and 22 men without thyroid pathology, thyroiditis, localized lesions,calcium metabolic disease, not taking these drugs affect thyroid. Identify Region Of Interest (ROI ) for thyroid tissue depth of 1.2 cm, for respectively stenocleidomastoid muscle depth of 0,6 cm.The result measured by two observers with different levels of experience performed independently and blindly. Results: Average velocity of shear wave in the healthy’s thyroid tissue is 1,47 ± 0,41m/s. There is no statistically significant difference in shear wave velocity between two gender and age (p > 0,05). Average velocity of shear wave in the healthy’s stenocleidomastoid muscle is 1,42 ± 0,32m/s. There is no statistically significant difference in shear wave velocity between three age groups but there is statistically significant difference in shear wave velocity between two gender (p < 0,05).In term of interobserver results, no statistically significant difference in shear wave velocity obtained by two observers with different levels of experiences (P > 0,05). Conclusions : The results of this study show that shear wave velocity measurement in healthy’s thyroid of women (1,50 ± 0,41 m/s) was significantly higher than in men but there is no statistically significant (p > 0,05). In contrary, average velocity of shear wave in the healthy’s stenocleidomastoid muscle of men (1,54 ± 0,29 m/s) was higher than in women (1,33 ± 0,32 m/s) statistically significant ( p < 0,05). Thyroid tissue elastogram shows the structure of the tissue is quite soft, homogeneous and smaller B-mode image. ARFI can be performed in the thyroid tissue with reliable results.
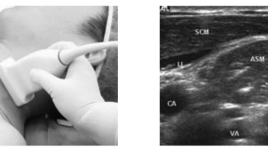
Bước đầu nghiên cứu đặc điểm giải phẩu siêu âm đám rối thần kinh cánh tay vùng cổ
02/04/2020 17:30:59 | 0 binh luận
Initial study of anatomical ultrasound imaging of neck region brachial plexus SUMMARY: Objective: To describe the sonographic appearance of the normal brachial plexus and to suggest the suitable region for brachial plexus blockade under the guidance of ultrasound. Subjects and methods: 15 patients whose age varies from 32 to 83, hospitalized at ultrasonography department in Hue Central Hospital from 3/2012 to 4/2012. The subjects had beenultrasound scanned to locate the brachial plexuses. Their sonographic appearances and relationship with surrounding structures then were described and cross- sectional description were studied. Results: Of all 15 subjects, brachial plexus appearances were well vizualized at interscalene and supraclavicular region. At infraclavicular region however, the same result occured in only 3 subjects, equaling to 20%. The brachial plexus was visualized as a chain of hypoechoic nodules representing the trunks at interscalene region, a cluster of hypoechoic nodules representing the divisions at the supraclavicular and hyperechoic nodules representing the cords of which diameters decrease from roots to nervous fiber. Brachial plexus’s location is nearer from skin at its roots and deeper towards the fiber end. Antomically, brachial plexus relates to stenocleidomastoid muscle, anterior scalene muscle, interscalene, subclavian artery at superior thoracic aperture and lung membrane at subclavian region. The brachial plexus that locates at interscalene region is the most suitable for brachial plexus desensitilization with ultrasound guide method for better efficiency and safety. Conclusion: Initial studies of anatomical ultrasound imaging of the brachial plexus in the neck region result in two conclusions. Firstly, high resolution ultrasound (7-10 MHz probe) allows clear description of brachial plexus from roots to divisions, and clear description of cords in about 20% of cases. Lastly, the interscalene region is the best place for neck region brachial plexus desensitilization with ultrasound guide method for its highest possibility of success and least complication.
Ứng dụng sinh thiết( ST) lõi trong chẩn đoán ung thư (UT) gan dưới hướng dẫn siêu âm
02/04/2020 14:54:27 | 0 binh luận
Core biopsies for focal hepatic lesions by ultrasound guidance SUMMARY: All percutaneous US-guided biopsies for focal liver lesions performed in 37 patients. Among the 54 lesions for which the 18- gauge cutting needle was used, core biopsy was performed once in 23 lesions (62.2%), twice in 11 lesions (29.7%), and three times in 3 lesions (8.1%). The length of tissue cores was above 1cm in 44 lesions (81.5%). Three of the 54 specimens (5.6%) fragmented when placed in formalin. The common complications were 2.7%. No major complications were observed. The core biopsy specimen was sufficient for diagnosis in 36 patients (97.3%) and was insufficient in one. Histologic examination revealed various types of H.C.C (62.2%), peripherial cholangiocarcinoma (2.7%), secondary malignant tumors (8.1%), benign tumors (24.3%). There was high concordance between the histologic and cytologic results (Kappa = 0.579, p < 0.05); and very high concordance between the core biopsy and post surgery histologic results (Kappa = 0.942, p < 0.05). Key words: core biopsy, focal hepatic lesions, HCC, cholangiocarcinoma.
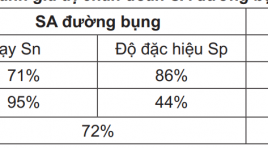
So sánh giá trị của siêu âm đường bụng và đường âm đạo trong chẩn đoán thai ngoài tử cung
02/04/2020 14:17:53 | 0 binh luận
Evaluated comparison of transvaginal and abdominal ultrasound in diagnosis of ectopic pregnancy SUMMARY: Purpose: To compare the value of abdominal and transvaginal ultrasound in diagnosis of ectopic pregnancy. Materials and methods: Cross-section and descriptive study was underwent on 140 patients who were suspected of ectopic pregnacies, from June 2009 to June 2010. All of them were done both abdominal and transvaginal ultrasound. The value of each modality was analysed base on gold standard of histopathology. Results: 140 cases of suspected ectopic pregnancy in which 110 were ectopic pregnancies, 10 viable intrauterine pregnancies, 19 abortions and 1 hydatidiform mole. The suggestive abdominal ultrasound diagnosis of ectopic pregnancy has Sn 71%, Sp 86%, PPV 95% and NPV 44%, those of transvaginal ultrasound has Sn 92.7%, Sp 96.6%, PPV 98% and NPV 96,6%. Conclusion: Transvaginal ultrasound has a primary role in the diagnosis of ectopic pregnancy
Bạn Đọc Quan tâm
Sự kiện sắp diễn ra
Thông tin đào tạo
- Những cạm bẫy trong CĐHA vú và vai trò của trí tuệ nhân tạo
- Hội thảo trực tuyến "Cắt lớp vi tính đếm Photon: từ lý thuyết tới thực tiễn lâm sàng”
- CHƯƠNG TRÌNH ĐÀO TẠO LIÊN TỤC VỀ HÌNH ẢNH HỌC THẦN KINH: BÀI 3: U não trong trục
- Danh sách học viên đạt chứng chỉ CME khóa học "Cập nhật RSNA 2021: Công nghệ mới trong Kỷ nguyên mới"
- Danh sách học viên đạt chứng chỉ CME khóa học "Đánh giá chức năng thất phải trên siêu âm đánh dấu mô cơ tim"